
Shiyang Liu#, Weifeng Liu#, Nengchao Luo*
ChemSusChem, 2025, DOI: 10.1002/cssc.202500208
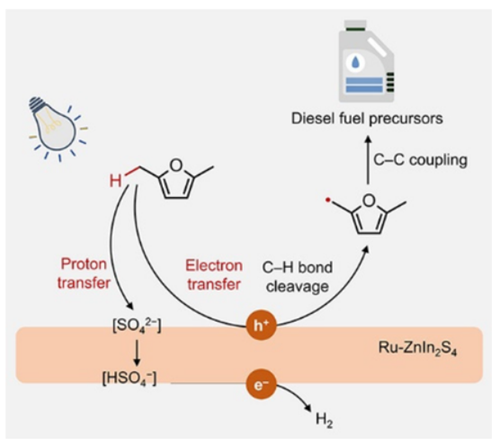
Photocatalytic C-C coupling of 2,5-dimethylfuran (DMF) derived from biomasses coproduces drop-in fuels and green hydrogen with a low-carbon footprint. However, the high reaction barrier for C-H bond breaking and uphill overall reaction lead to the slow kinetics of DMF coupling. Here, we reveal that [HSO4−] and water can collaboratively promote the rate-limiting step of the C-H bond breaking on the Ru-ZnIn2S4 catalyst. An in-depth study suggests that water mediates hole transfer to the C-H bond while [HSO4−] facilitates electron extraction, thus promoting electron and proton transfer on the Ru-ZnIn2S4 surface. This work puts forward new insight and strategy for photocatalytic C-C coupling for the synthesis of biofuels.
Ping Jin, Pu Guo, Nengchao Luo,* Hui Zhang, Chenwei Ni, Ruotian Chen, Wei Liu, Rengui Li, Jianping Xiao, Guoxiong Wang, Fuxiang Zhang, Paolo Fornasiero,* Feng Wang*
Science, 2025, DOI: 10.1126/science.adq3445.
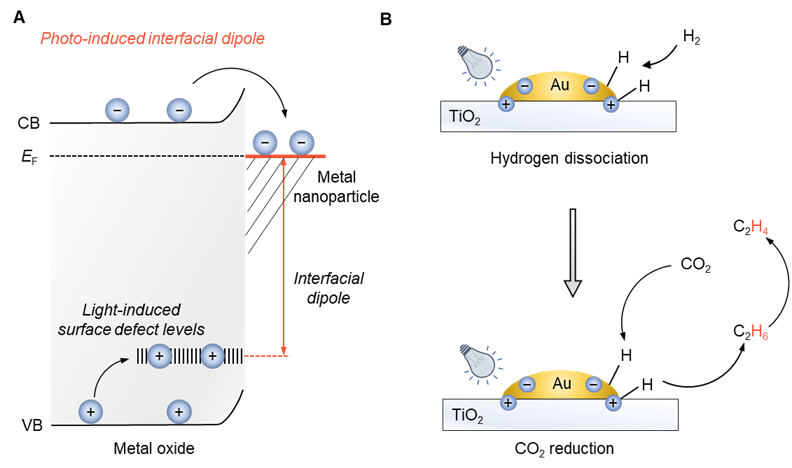
Producing olefins by CO2 hydrogenation is a longstanding goal. The usual products are C2+ mixtures because the critical step of heterolytic H2 dissociation at high temperatures complicates selectivity control. Here we report that irradiating Au/TiO2 at 365 nm induces heterolytic H2 dissociation at ambient temperature. This process likely relies on interfacial electric dipoles from photogenerated electrons and holes situated on the metallic Au nanoparticles and interfacial Au–O–Ti scaffolds. The heterolytic H2 dissociation is further promoted by light-induced coating of Au nanoparticles with a TiOx layer. The resulting nucleophilic hydrogen species reduce CO2 to ethane in >99% yield under light irradiation in a flow apparatus. Furthermore, cascading with a subsequent photocatalytic ethane dehydrogenation generates ethylene in >99% yield over 1500 h of irradiation.
Yifan Yang, Jianhan Wu, Zhendong Luo, Xiuquan Jia*, Guibin Jiang*, and Feng Wang*
J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c06438
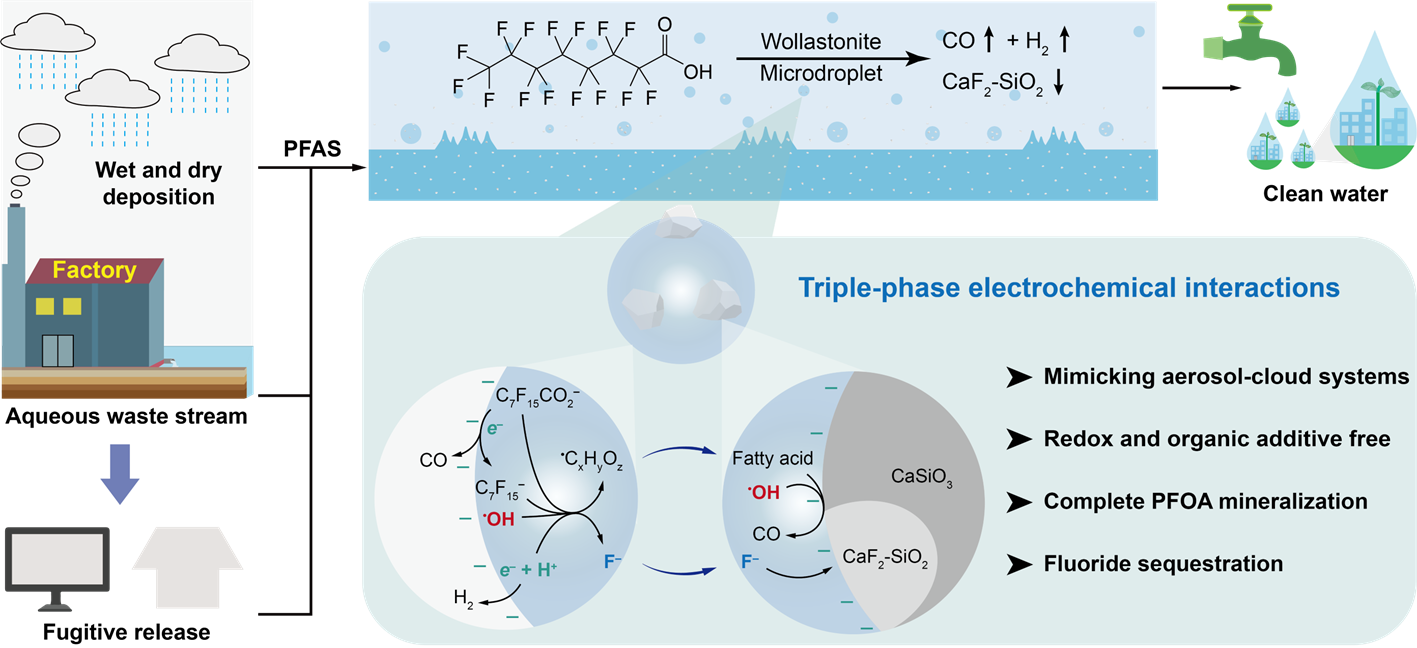
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are pervasive contaminants in water resources. Current nonthermal defluorination strategies face critical limitations including incomplete mineralization, residual fluoride ion and so on, thereby hindering compliance with water quality standards. Here, we report that wollastonite-bearing microdroplets prioritize defluorination over C-C scission through liquid-solid-gas triple-phase contact electrification, enabling complete perfluorooctanoic acid mineralization with hardly detectable shorter-chain PFAS byproducts. Due to the Si-F-Ca structure forming in microdroplet-mediated weathering process of wollastonite, fluoride immobilization with negligible leaching could be realized. This work reveals that atmospheric clouds containing mineral particles intrinsically exhibit a self-cleansing capacity toward PFAS contaminants, advancing cloud-inspired interfacial engineering for next-generation water purification systems.
Yafei Liang1, Yuji Qi1, Mingli Bi, Zhen Shi, Junju Mu, Shushuang Li, Jian Zhang, Yehong Wang*, Feng Wang
J. Energy Chem., 2025, 106, 832-841. DOI: 10.1016/j.jechem.2025.02.061
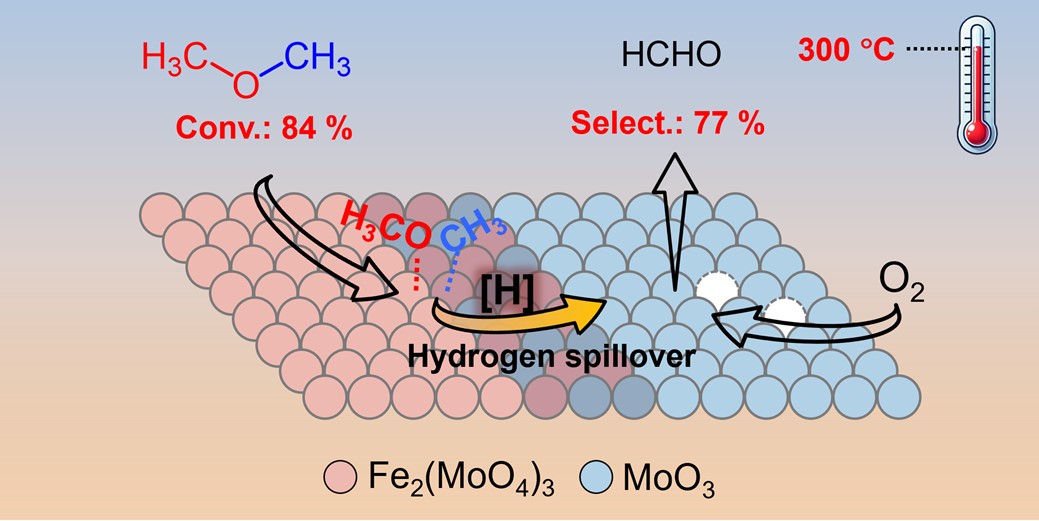
The selective oxidation of dimethyl ether (DME) represents a promising approach to producing high-concentration formaldehyde with low energy consumption. However, there is still a lack of catalysts achieving satisfactory conversion of DME with high selectivity for formaldehyde under mild conditions. In this work, an efficient iron-molybdate (FeMo) catalyst was developed for the selective oxidation of DME to formaldehyde. The DME conversion of 84% was achieved with a superior formaldehyde selectivity (77%) at 300 °C, which was superior to all previously reported results. Combined comprehensive characterizations demonstrated that FeMo catalyst was composed of active Fe2(MoO4)3 and MoO3 phases, and there was an interaction between them, which contributed to the efficient DME dissociation and smooth hydrogen spillover. These findings offer valuable insights for the development of efficient catalysts and elucidating the reaction mechanism involved in the oxidation of DME to formaldehyde.
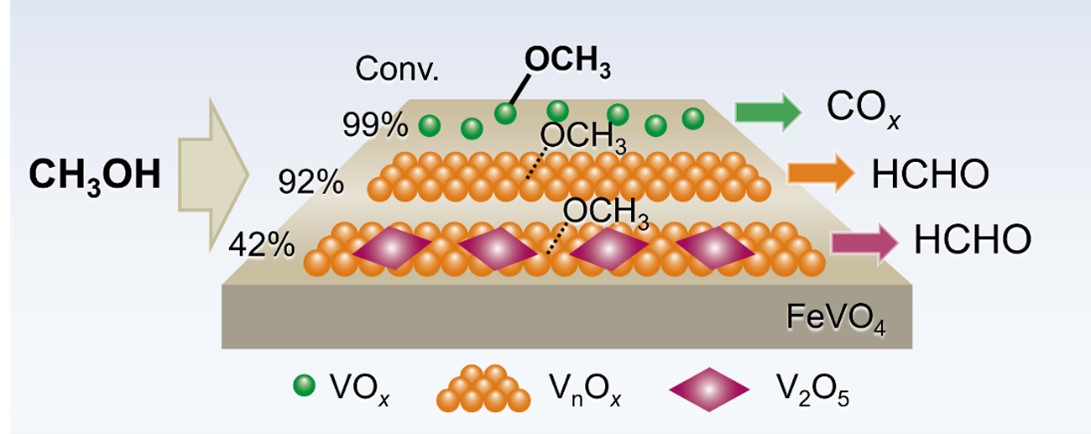
Yujie Zhan, Chengqin Zhong, Mingli Bi, Yafei Liang, Yuji Qi, Jiaqi Chen, Jiaxu Liu, Xindang Zhang, Shuai Zhang, Yehong Wang*, Feng Wang
Chin. J. Catal, 2025. (DOI: 10.1016/S1872-2067(24)60279-2.)
Iron-Vanadium (FeV) catalysts exhibit unique activity for methanol oxidation to formaldehyde, while identifying active sites is challenging due to their complex compositions. In this work, FeV catalysts with various compositions, such as FeVO4 with isolated VOx, low-polymerized VnOx, and crystalline V2O5, were prepared and tested for methanol oxidation. A FeV1.1 catalyst, containing FeVO4 and low-polymerized VnOx, achieved 92.3% methanol conversion and 90.6% formaldehyde selectivity, comparable to conventional iron-molybdate catalysts. CH3OH-IR, O2 pulse, and control experiments revealed a synergistic effect between FeVO4 and low-polymerized VnOx, enhancing oxygen supply and binding strengths for formaldehyde intermediates. This study deepens the understanding of FeV structures and provides guidelines for methanol oxidation to formaldehyde.
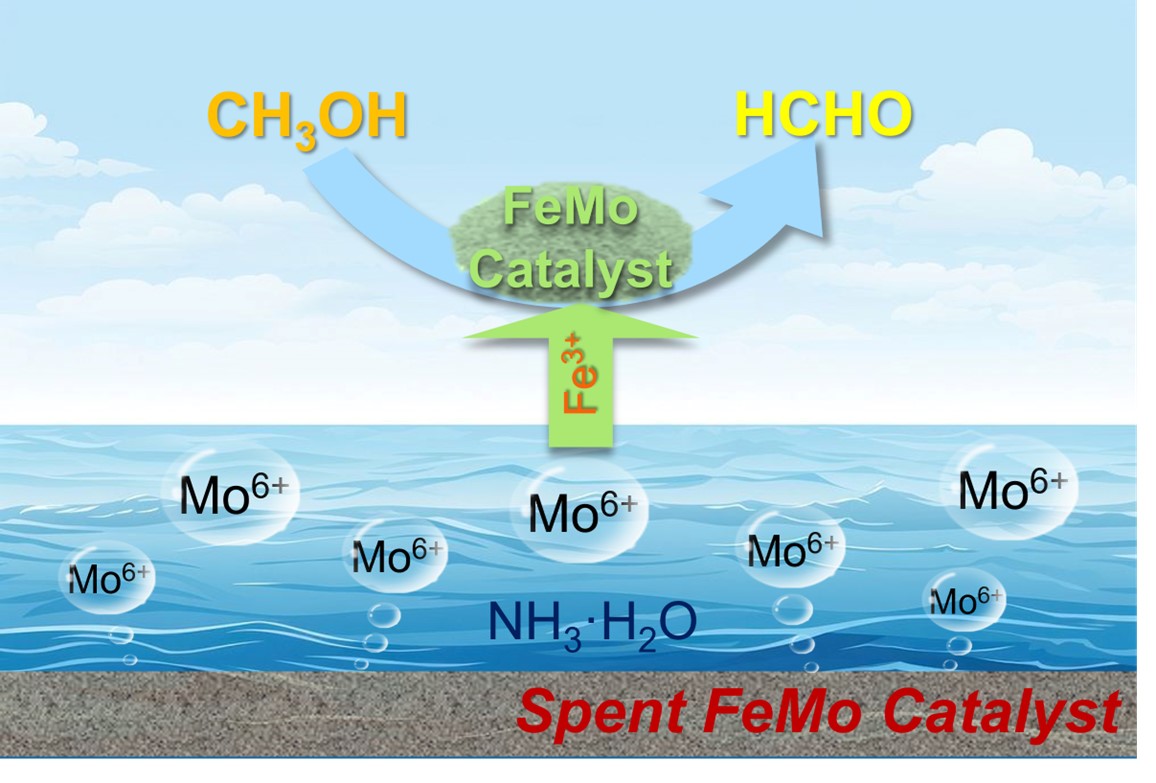
Yuji Qi, Yafei Liang, Mingli Bi, Shushuang Li, Zhen Shi, Jian Zhang, Xindang Zhang, Shuai Zhang, Yehong Wang* and Feng Wang
Industrial & Engineering Chemistry Research
The FeMo catalyst is widely used for methanol oxidation to produce formaldehyde, but its lifetime is limited by molybdenum loss. Spent catalysts, containing significant molybdenum, are typically discarded. This study demonstrates a method to recover molybdenum from spent FeMo catalysts using ammonia leaching, achieving 95.3% efficiency. Analysis shows spent catalyst contains MoO₃, Fe₂(MoO₄)₃, and FeOₓ. Leaching involves MoO₃ dissolution at pH <6.3, followed by Fe₂(MoO₄)₃ leaching at higher pH, with Mo existing as Mo₃O₁₀²⁻, MoO₄²⁻, or Mo₇O₂₄⁶⁻ depending on pH. Recovered Mo was used to prepare new FeMo catalysts, achieving 99.9% methanol conversion and 89.3% formaldehyde selectivity, matching industrial standards. The regenerated catalyst showed excellent stability over 510 hours. This work offers a potential pathway for large-scale recovery and reuse of spent FeMo and other molybdenum-containing catalysts.

Genheng Li, Dalie An, Xukai Zhou,* Feng Wang
ACS Catal. 2025, 15, 7800–7809 (DOI: 10.1021/acscatal.5c00937
This study presents an efficient strategy for synthesizing ε-caprolactam from succinic acid derivatives through a two-stage process integrating photocatalytic decarboxylative alkylation and thermocatalytic reductive cyclization. By incorporating Ru particles on TiO2, the photocatalytic decarboxylation of monoethyl succinate and subsequent Giese addition into acrylonitrile were achieved (ambient pressure and temperature, 365 nm irradiation), followed by the thermocatalytic hydrogenation of ethyl 5-cyanopentanoate (2 MPa H2, 180 °C) to form ε-caprolactam, with yields of 81 and 70%, respectively. This innovative two-in-one catalytic system eliminates the need for additional catalysts and offers reusability, reducing costs and enhancing practical applicability. The mechanism was comprehensively studied, providing insights into extending this approach to other reactions.
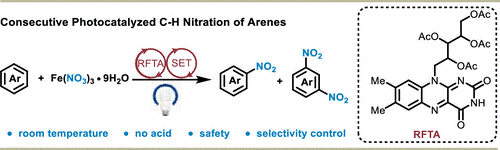
Da-Lie An, Chao Xu, Manman Wang,* Qinghai Shu, Xukai Zhou*
J. Org. Chem. 2025, 90, 4313–4324 (DOI: 10.1021/acs.joc.5c00024)
Concerned with traditional nitration methods requiring high temperatures, strong acids, or oxidizing agents, we developed an acid-free, selective photocatalytic nitration method using riboflavin tetraacetate and Fe(NO3)3·9H2O under visible light. This method efficiently nitrates various arenes and bioactive molecules with high selectivity and functional group tolerance, offering a sustainable alternative to traditional nitration techniques.
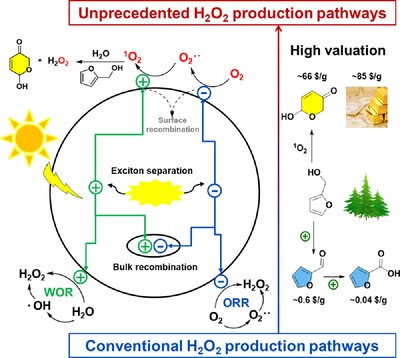
Qiang Xue, Hanxi Li, Peng Jin, Xukai Zhou,* Feng Wang*
Angew. Chem. Int. Ed. 2025, e202423368. (DOI: 110.1002/anie.202423368)
A strategy of photocatalytic valorization of furfuryl alcohol (FFA) coupled with the efficient co-production of H2O2 is reported, enabled by covalent organic frameworks (COFs) induced, 1O2-participated Achmatowicz rearrangement. This study introduces polyimide-based COF-N0-3 with tailored nitrogen content, representing an unprecedently efficient platform for 1O2 production. Remarkably, reducing the nitrogen content of the COF enhances 1O2 production, significantly boosting the H2O2 generation rate. In FFA, the primary pathway for H2O2 production is Achmatowicz rearrangement, achieving a rate ten times higher than that reliant on oxygen reduction reaction in pure water, reaching 4549 µmol g⁻¹ h⁻¹. Mechanism studies revealed 1O2 selectively engaged FFA, bypassing hole oxidation to trigger the Achmatowicz rearrangement, producing valuable 6-hydroxy-(2H)-pyranone with 99% conversion and 92% selectivity.
Siqi Li, Yuda Zhang, Qiang Guo, Yafei Liang, Yuji Qi, Xiaowei Zhao, Yehong Wang*, Feng Wang
Chem. Eng. J., 2025, 508, 161144
The ethanol dehydrogenation condensation to ethyl acetate is a promising pathway, however, limited to low ethyl acetate selectivity due to the various side-reactions catalyzed by acid-base sites. Here, an efficient CuZnZrO solid solution catalyst was developed, and a superior ethyl acetate selectivity of 95.7% was achieved with a comparable ethanol conversion of 50.3%. The balance of acid-base aroused from zinc (Zn) doping into the CuZrO matrix contributed to its excellent catalytic performance. It was also noteworthy that the result of ∼2200 h life-test suggested a remarkable stability of CuZnZrO solid solution catalyst. This work offers a highly efficient catalytic system for the selective production of ethyl acetate from ethanol.

Xueyuan Wang, Xueshang Xin, Lunqiao Xiong, Jianlong Yang, Tieou Wang, Yang Yang, Zhipeng Huang, Nengchao Luo*, Junwang Tang*, Feng Wang
Angew. Chem. Int. Ed., 2025, DOI: 10.1002/anie.202420606
Hydroxy radical (•OH) is a prestigious oxidant that allows the cleavage of strong chemical bonds of methane but is untamed, leading to over-oxidation of methane and waste of oxidants, especially at high methane conversion. Here, we managed to buffer •OH in an aqueous solution of photo-irradiated Fe3+, where •OH almost participates in methane oxidation. Due to the interaction between Fe3+ and SO42−, the electron transfer from OH− to excited-state Fe3+ for •OH generation is retarded, while excessive •OH is consumed by generated Fe2+ to restore Fe3+. When combined with a Ru/SrTiO3:Rh photocatalyst, the buffered •OH converts methane to C2+ hydrocarbons and H2 with formation rates of 246 and 418 μmol h−1, respectively. The apparent quantum efficiency reaches 13.0 ± 0.2%, along with 10.2% methane conversion and 81% C2+ selectivity after 80 hours of reaction. Overall, this work presents a strategy for controlling active radicals for selective and efficient photocatalysis.

Ping Wu, Fangming Du, Qiang Xue, Hanxi Li, Ming-Chen Fu, Xukai Zhou,* and Feng Wang
Adv. Funct. Mater. 2025, 2420941. (DOI: 10.1002/adfm.202420941)
In this paper, three gold(I) cyclic trinuclear complexes (Au-CTCs) based metal–organic frameworks (MOFs) are prepared, exhibiting good photothermal conversion efficiency and H2O2 evolution rates. The production rates of H₂O₂ can reach as high as 51,987 µm g⁻¹ h⁻¹, surpassing the performance of most reported MOFs. The thermal-assisted photocatalytic mechanism is comprehensively studied by transient photocurrent response, electrochemical impedance, electron paramagnetic resonance, rotating ring disk electrode test and among others, demonstrating thermal energy can enhance the mobility of photogenerated carriers and apparent quantum yield, regulate the reactive species ratio and H2O2 selectivity, reduce the apparent activation energy of photocatalysis, and improve mass transfer rates, thereby accelerating the reaction process. This study offers new insights into the thermal-assisted photocatalytic production of H2O2.

Hao Han+, Qiang Xue+, Jiehua Ding, Jianyu Han, Hanxi Li, Yougui Li,* Xukai Zhou*
ACS Appl. Polym. Mater. 2025, 7, 3, 1338–1346. (DOI: 10.1021/acsapm.4c03047)
The classical organic dyes anthracene, acridine, and phenazine, as building blocks in the construction of three covalent organic frameworks (COFs), are presented for comprehensive comparison studies. Employing a sophisticated atomic-level skeleton-editing strategy through the precise adjustment of nitrogen content enables the regulation of intrinsic properties across macro- to micro-scales. In application experiments, photocatalytic hydrogen evolution (PHE) is studied as a model reaction. The results suggest that the anthracene-based COF (COF-Ant), with the strongest electron-donating ability, achieves the highest PHE rate among the three COFs at 2493 μmol·g–1·h–1. Additionally, the uniformity of the three COFs is highlighted, including shared features such as organic dyes and imine linkages, resulting in wide visible absorption, a narrow band gap, and topological features.