

Huifang Liu, Jianyu Han, Qitian Huang, Hongwei Shen, Lijun Lei, Zhipeng Huang, Zhixin Zhang, Zongbao K. Zhao*, Feng Wang*
Ind. Eng. Chem. Res., 2020, 59, 17440-17450
Catalytic hydrotreating of renewable oils is a promising way to get diesel alkanes, with the use of microbial lipids further facilitating scalable production of green diesel. The core issue of triglyceride hydrodeoxygenation (HDO) research is still to develop efficient catalysts under mild reaction conditions. Herein, we demonstrate that the heteropolyacid (HPA)-modified SiO2 supported Pd catalysts showed high activity for the HDO reaction of methyl stearate and soybean oil to diesel alkanes (C15∼C18, >90 wt % at 200 °C). Characterizations show that the Brønsted acid sites introduced by grafting heteropolyacid on SiO2 and metal sites of small Pd particles contribute to the high HDO activity of this bifunctional catalyst. Additionally, lipids produced by Rhodosporidium toruloides Y4 from glucose or corn stover were also transformed to about 75 wt % of diesel-range alkane products......
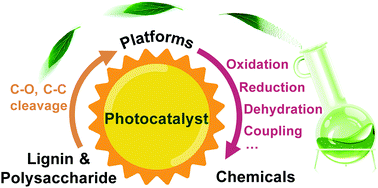
Xuejiao Wu, Nengchao Luo, Shunji Xie*, Haikun Zhang, Qinghong Zhang, Feng Wang*, Ye Wang*
Chem. Soc. Rev., 2020, 49, 6198-6223
As the largest renewable carbon resource, lignocellulosic biomass has great potential to replace fossil resources for the production of high-value chemicals, in particular organic oxygenates. Catalytic transformations of lignocellulosic biomass using solar energy have attracted much recent attention, because of unique reactive species and reaction patterns induced by photo-excited charge carriers or photo-generated reactive species as well as the mild reaction conditions, which may enable the precise cleavage of target chemical bonds or selective functionalisation of specific functional groups with other functional groups kept intact. Here, we present a critical review on recent advances in the photocatalytic transformation of lignocellulosic biomass with an emphasis on photocatalytic cleavage of C–O and C–C bonds in major components of lignocellulosic biomass, including polysaccharides and lignin, and the photocatalytic valorisation of some key platform molecules......
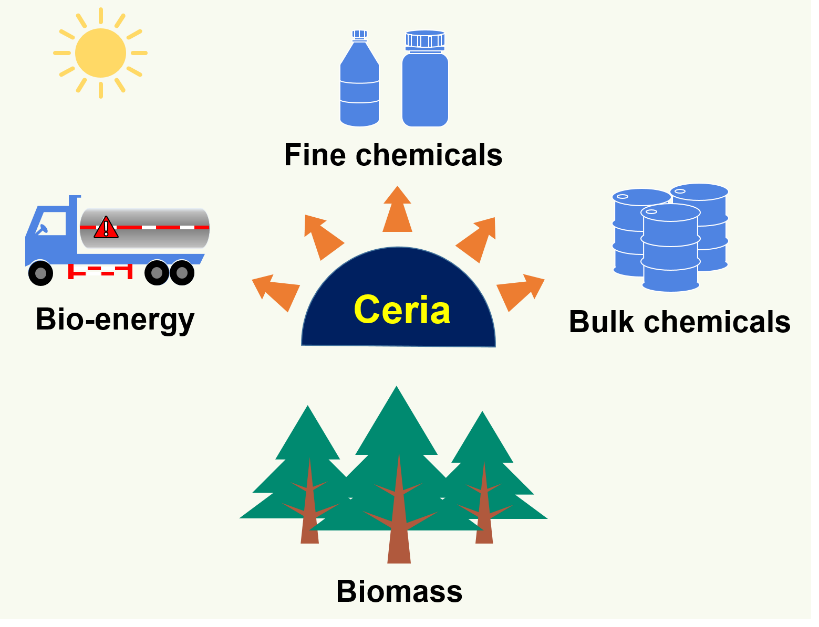
Lijun Lei, Yehong Wang, Zhixin Zhang, Jinghua An, Feng Wang*
ACS Catal., 2020, 10, 8788-8814
In the past two decades, on account of the energy and environmental crisis brought by the decline in fossil resources, price volatility, and climate change, the high-value utilization of biomass feedstocks has gradually attracted widespread attention. The reversible Ce3+/Ce4+ redox pairs and the existence of oxygen vacancies improve its redox ability and thus catalytic activity. Besides, the acid-base properties enable its use in acid-base catalytic reactions. The strength or concentration of acid-base sites is tailorable. The water-tolerance character is unique and thus can be employed in the conversion of dilute aqueous biomass solutions. In this perspective, we summarize the latest research progress in the high-value utilization of biomass feedstocks, including biomass raw materials, platform molecules originated from biomass as well as its derivatives and downstream chemicals over pure CeO2, doped-CeO2 and CeO2 supported metal catalysts.
Hongji Li‡, Meijiang Liu‡, Huifang Liu, Nengchao Luo, Chaofeng Zhang, Feng Wang*
ChemSusChem, 2020, 13, 4660-4665
Introducing amines/ammonia into lignin cracking will allow novel bond cleavage pathways. Herein, a method of amines/ammonia-mediated bond cleavage in oxidized lignin β-O-4 models was studied using a copper catalyst at room temp., demonstrating the effect of the amine source on the selectivity of products. For primary and secondary aliph. amines, lignin ketone models underwent oxidative Cα-Cβ bond cleavage and Cα-N bond formation to generate arom. amides. For ammonia, the competition between oxygen and ammonia detd. the selectivity between Cα-N and Cβ-N bond formation, generating amides and α-keto amides, resp. For tertiary amines, the lignin models underwent oxidative Cα-Cβ bond cleavage to benzoic acids. Control expts. indicated that amines act as nucleophiles attacking at the Cα or Cβ position of the oxidized β-O-4 linkage to be cleaved. This study represents a novel example that the breakage of oxidized lignin model can be regulated by amines with a copper catalyst.
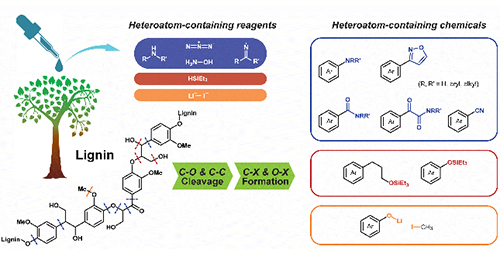
Hongji Li, Anon Bunrit, Ning Li, Feng Wang*
Chem. Soc. Rev., 2020, 49, 3748-3763
Lignin, the most abundant aromatic polymer in nature, enables sustainable supply of miscellaneous aromatics as green fuels and chemicals. Obtaining the value-added aromatics from lignin, though subjected to enormous research efforts, mainly relies on depolymerization induced by activated hydrogen species or oxygen species, delivering hydrocarbons and oxygenates. The future bio-refinery demands a broad spectrum of fine chemicals, especially those containing elements other than C, H and O. Heteroatom-containing compounds have emerged as powerful reagents to participate in the bond cleavage in lignin; meanwhile, the obtained heteroatom-containing aromatics, which could be used as dye precursors, pharmaceutical precursors, hydrogen storage materials, etc., extend the application of lignin-derived products...

Hongji Li, Zhuyan Gao, Lijun Lei, Huifang Liu, Jianyu Han, Feng Hong, Nengchao Luo, Feng Wang*
Green Chem., 2020, 22, 3802-3808
A mild method of photocatalytic deoxygenation of aromatic ketones to alkyl arenes was developed, which utilized alcohols as green hydrogen donors. No hydrogen evolution during this transformation suggested a mechanism of direct hydrogen transfer from alcohols. Control experiments with additives indicated the role of acid in transfer hydrogenolysis, and catalyst characterization confirmed a larger number of Lewis acidic sites on the optimal Pd/TiO2 photocatalyst. Hence, a combination of hydrogen transfer sites and acidic sites may be responsible for efficient deoxygenation without additives. The photocatalyst showed reusability and achieved selective reduction in a variety of aromatic ketones.
Jinghua An, Yehong Wang, Zhixin Zhang, Jian Zhang, Feng Wang*
Chin. J. Catal., 2020, 41(6), 963-969
Hydroalkoxycarbonylation of olefins has been considered to be one of the most attractive methods to synthesize esters. Controlling the regioselectivities of linear esters (L) and branched esters (B) is a challenging project for researchers working in this reaction. Although most of the attention has been paid to control the regioselectivity through ligand design in homogeneous catalytic sy...
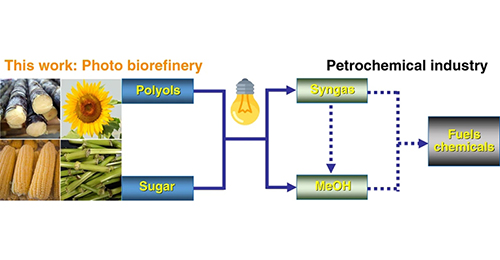
Min Wang, Meijiang Liu, Jianmin Lu, Feng Wang*
Nat. Commun., 2020, 11, 1083
We report the conversion of biomass-derived polyols and sugars into methanol and syngas (CO+H2) via UV light irradiation under room temperature, and the bio-syngas can be further used for the synthesis of methanol. The cellulose and even raw wood sawdust could be converted into methanol or syngas after hydrogenolysis or hydrolysis pretreatment. We find Cu dispersed on titanium oxide nanorod (TNR) rich in defects is effective for the selective C−C bond cleavage to methanol. Methanol is obtained from glycerol with a co-production of H2. A syngas with CO selectivity up to 90% in the gas phase is obtained via controlling the energy band structure of Cu/TNR.

Zhipeng Huang‡, Zhitong Zhao‡, Chaofeng Zhang, Jianmin Lu, Huifang Liu, Nengchao Luo, Jian Zhang, Feng Wang*
Nat. Catal., 2020, 3, 170-178
Here we demonstrate that photogenerated radicals can be rapidly terminated by surface hydrogen species during photocatalytic decarboxylation of fatty acids on a hydrogen-rich surface that is constructed by the interactions between H2 and Pt/TiO2 catalyst, thereby greatly inhibiting oligomerization; Cn–1 alkanes can therefore be obtained from bio-derived C12–C18 fatty acids in high yields (≥90%) under mild conditions (30 °C, H2 pressure ≤0.2 MPa) and 365 nm light-emitting dode irradiation. Industrial low-value fatty acid mixtures (namely, soybean and tall oil fatty acids) can be transformed into alkane products in high yields (up to 95%). Our research introduces an efficient biomass-upgrading approach that is enabled by subtle control of the radical intermediate conversion on a heterogeneous surface.

Kaiyi Su, Huifang Liu, Bin Zeng, Zhixin Zhang, Nengchao Luo, Zhipeng Huang, Zhuyan Gao, Feng Wang*
ACS Catal., 2020, 10(2), 1324-1333
Photocatalytic selective oxidation of hydrocarbons to oxygenated chemicals greatly relies on catalytic materials that show high efficiency of photogenerated holes and electrons separation and visible light absorption capacity. We, herein, report one facile calcination approach with ammonium chloride and melamine as the template to synthesize nitrogen-modified Nb2O5 nanomeshes material (Nb2O5-N), which exhibits a 37-fold reaction rate larger than its commercial counterpart in photocatalytic oxidation of toluene into benzaldehyde under visible light irradiation. The reactivity is ascribed to an extended absorption spectrum within 700 nm by nitrogen modification. In addition, photocurrent response results suggest that a relaxation effect induced by nanomesh structure is beneficial for the separation of charge carriers for enhanced reactivity.
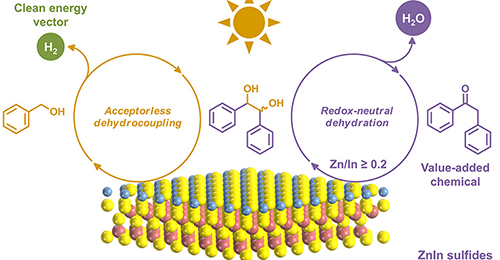
Nengchao Luo, Tingting Hou, Shiyang Liu, Bin Zeng, Jianmin Lu, Jian Zhang, Hongji Li, Feng Wang*
ACS Catal., 2020, 10(1), 762-769
Photocatalytic H2 evolution from organic feedstocks with simultaneous utilization of photogenerated holes achieves solar energy storage and coproduces value-added chemicals. Here we show visible-light H2 production from benzyl alcohol (BAL) with controllable generation of deoxybenzoin (DOB) or benzoin (BZ) through tandem redox reactions. Particularly, DOB synthesis circumvents the use of expensive feedstocks and environmentally unfriendly catalysts that are required previously. Under the irradiation of blue LEDs, the key of steering the major product to DOB rather than BZ is to decrease the conduction band bottom potentials of the ZnIn sulfide catalysts by increasing the Zn/In ratio, which results in the dehydration of intermediate hydrobenzoin (HB) to DOB proceeding in a redox-neutral mechanism and consuming an electron–hole pair. As a proof of concept, this method is used to synthesize DOB derivatives in gram scale.
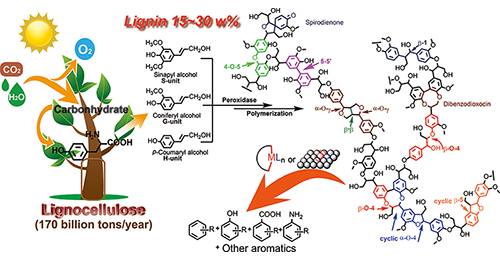
Chaofeng Zhang, Feng Wang*
Acc. Chem. Res., 2020, 53(2), 470-484
We present our recent studies on lignin's catalytic conversion to aromatic chemicals. First, we introduce our research on protolignin depolymerization via a fragmentation–hydrogenolysis process in alcohol solvents. Then, focusing on the catalytic cleavage of lignin C–C and C–O bonds, we shed light on a recapitulative adjacent functional group modification (AFGM) strategy for the conversion of lignin models. AFGM strategy begins with the adjacent functional group modification of the target C–C or C–O bond to directly decrease the bond dissociation enthalpy (BDE) of targeted bonds or generate new substrate sites to introduce the cleavage reagent for further conversion. Subsequently, on the basis of these two concepts from AFGM, we summarize our strategies on lignin depolymerization, which highlight the effects of lignin structure, catalyst character, and reaction conditions on the efficiency of strategies.

Huifang Liu, Hongji Li, Nengchao Luo, Feng Wang*
ACS Catal., 2020, 10(1), 632-643
Using vanadium catalysts under visible light, we selectively cleave the C–C bonds in β-1 and β-O-4 interlinkages occluded in lignin models and extracts by an oxidative protocol. Visible light irradiation triggered single electron transfer between the substrate and the catalyst, which further induced the selective Cα–Cβ bond cleavage and generated the final aromatic products through radical intermediates. Using this photocatalytic chemistry, the reactivity of lignin models and the selectivity of Cα–Cβ bond cleavage were significantly improved. More importantly, this protocol affords aromatic monomers through the fragmentation of organosolv lignins even at room temperature, indicating the potential of photocatalytic C–C bond cleavage of lignin linkages under ambient conditions.