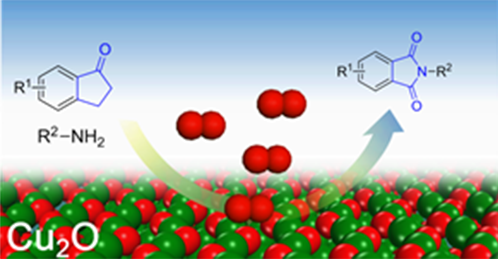
The cyclic imides are widely used in biological, medicinal, and polymer chemistry. They have been traditionally synthesized by heating dicarboxylic acids or anhydrides with an amine. In general, harsh thermal reaction conditions and activation reagents are mandatory. Recently, we report a new reaction for the synthesis of cyclic imide via oxidative coupling cyclic ketones with amines. Ketones and amines are widely available chemicals. It is promising method to synthesize various cyclic imide from ketones and amines. This work has been published online in the Angewandte chemie.
Article information: Min Wang, Jianmin Lu, Jiping Ma, Zhe Zhang, Feng Wang*. Cuprous Oxide Catalyzed Oxidative C-C Bond Cleavage for C-N bond formation: Synthesis of Cyclic Imides from Ketones and Amines. Angew. Chem. Int. Ed., 2015, DOI: 10.1002/anie.201508071. link
Abstract: Selective oxidative cleavage of a C-C bond offers a straightforward method to functionalize organic skeletons. Reported herein is the oxidative C-C bond cleavage of ketone for C-N bond formation over a cuprous oxide catalyst with molecular oxygen as the oxidant. A wide range of ketones and amines are converted into cyclic imides with moderate to excellent yields. In-depth studies show that both α-C-H and β-C-H bonds adjacent to the carbonyl groups are indispensable for the C-C bond cleavage. DFT calculations indicate the reaction is initiated with the oxidation of the α-C-H bond. Amines lower the activation energy of the C-C bond cleavage, and thus promote the reaction. New insight into the C-C bond cleavage mechanism is presented.